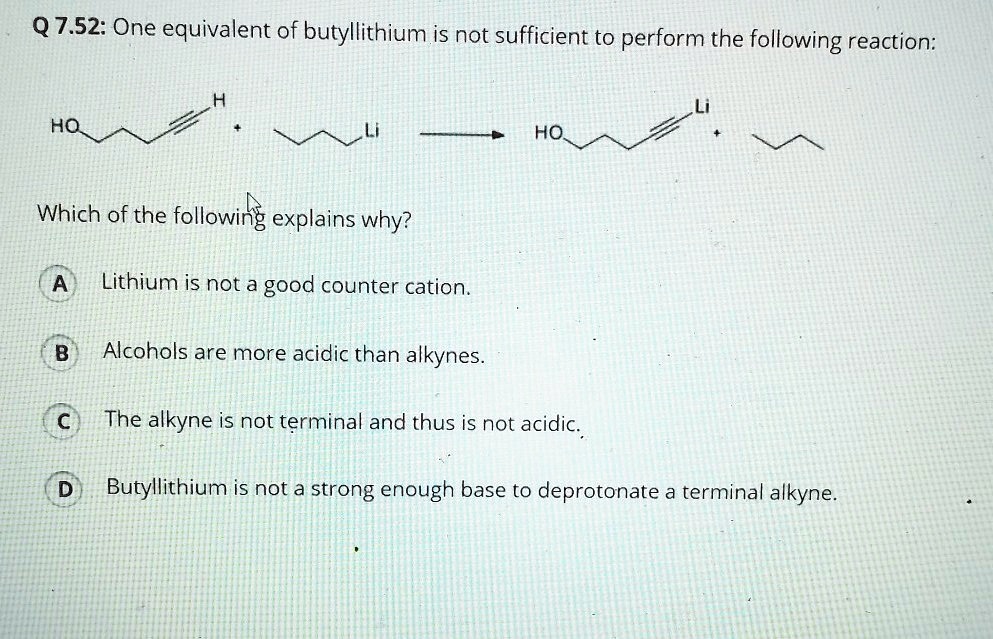
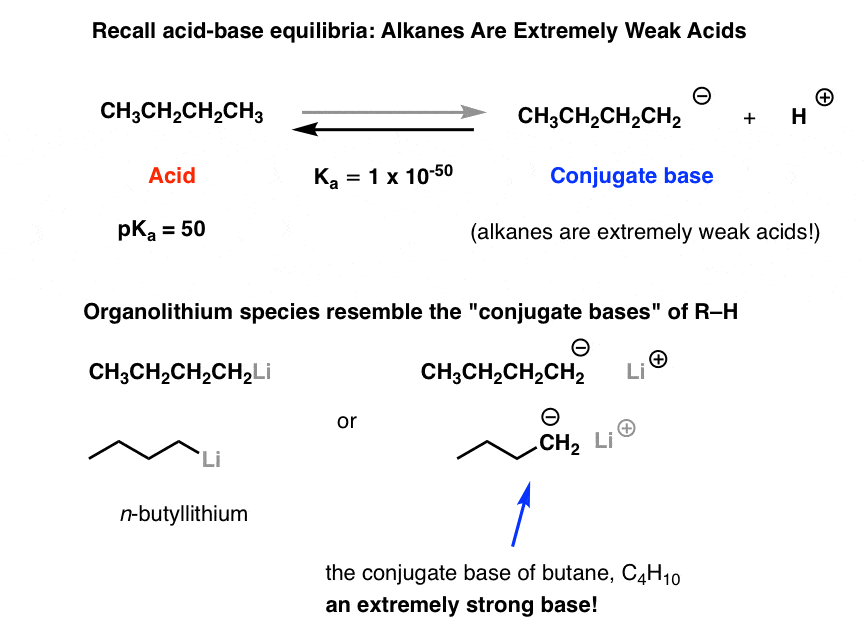
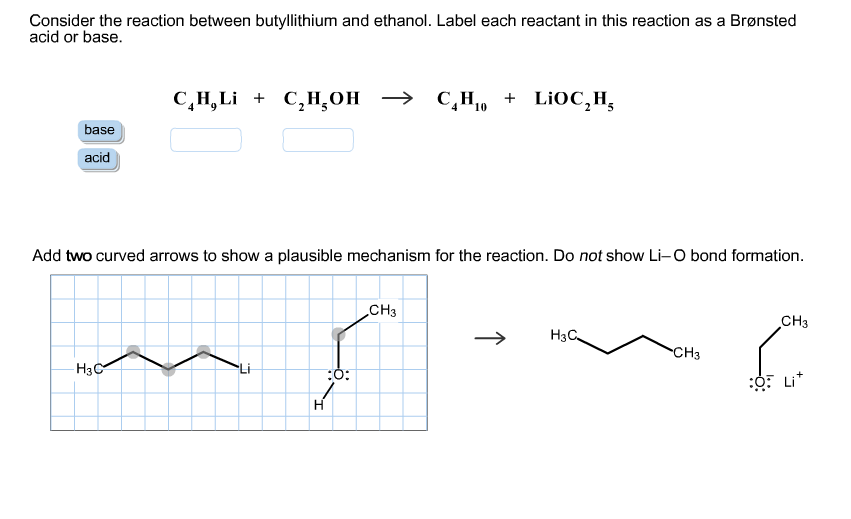
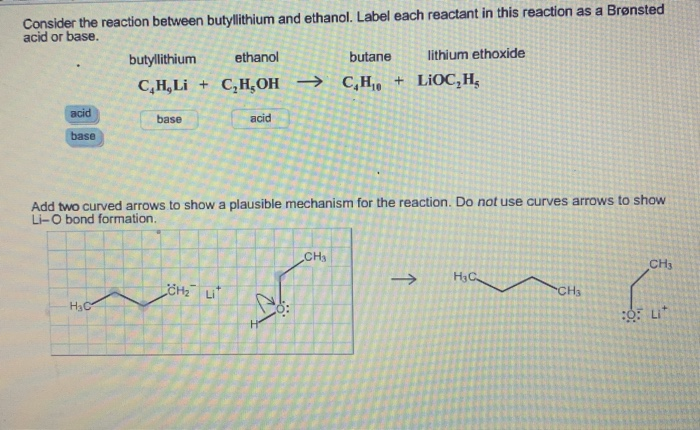
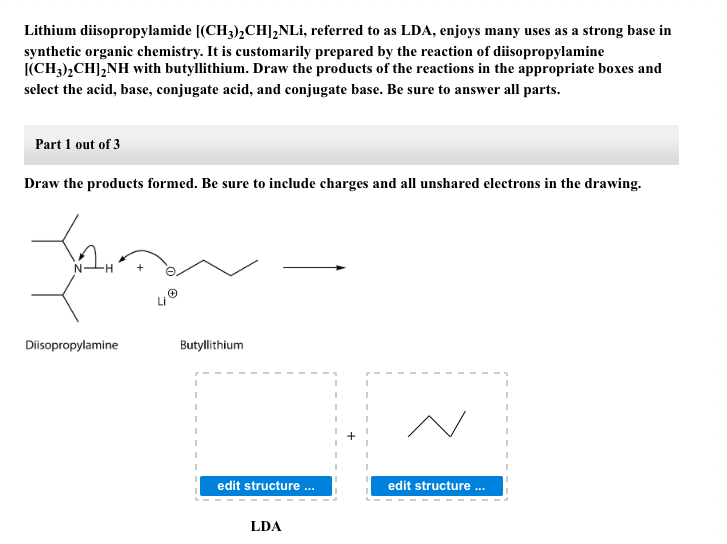
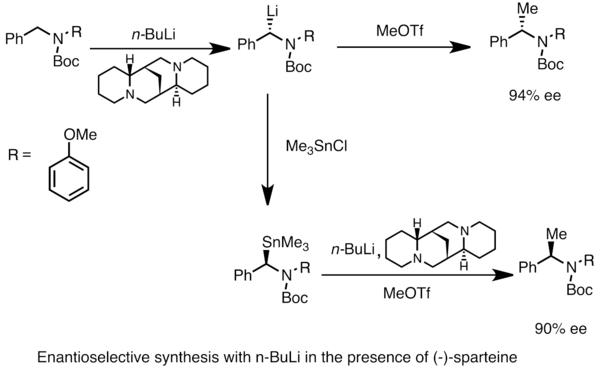
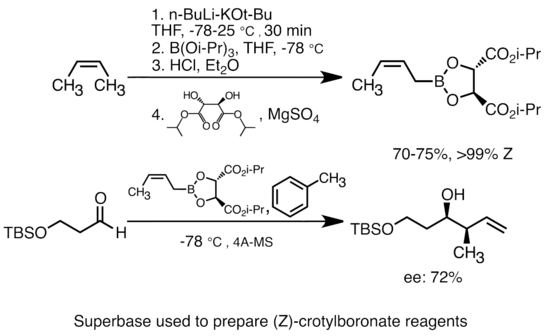
SOLVED: Match the species below to their appropriate, relative basic strength. butyllithium acetate alkoxide acetate Weakest Base alkoxide 2) Medium strength base 3) Strongest Base butyllithium

Acros Organics AC187540090 sec-Butyllithium, 1.3M solution in cyclohexane/hexane (92/8) (9g) from Cole-Parmer India

n-Butyllithium-promoted regioselective elimination of vicinal bis-triflate having an adjacent ether oxygen - ScienceDirect

n-Butyllithium/N,N,N',N'-Tetramethylethylenediamine-Mediated Ortholithiations of Aryl Oxazolines: Substrate-Dependent Mechanisms | Journal of the American Chemical Society

Ministry of Chemistry - n-#Butyllithium (abbreviated #nBuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as #polybutadiene or styrene-butadiene-styrene (#SBS). Also, it